EVIDENCE-BASED
Breakthroughs
EVIDENCE-
BASED
Breakthroughs
Transforming vascular care based on evidence-based decisions means utilizing clinical data that meets the highest standards. Robust clinical evidence program to establish new standards to transform vascular care. International, randomized, multi-center studies serve as the foundation for disruptive innovation.
Landmark BIOADAPTOR RCT
DynamX coronary bioadaptor is first device with RCT data demonstrating significantly better effectiveness in early return of vessel function and stabilization of plaques vs. state-of-the-art drug-eluting stent, the Resolute Onyx™ DES.
The BIOADAPTOR RCT is an international, single-blinded, randomized controlled (1:1) trial comparing a sirolimus-eluting bioadaptor with a contemporary zotarolimus-eluting stent in 445 patients. Both arms had large randomized multi-imaging modality subgroups of 100 patients powered to document standard stent effectiveness benchmarks, and the new effectiveness benchmarks of vessel motion and function. Data collection will continue through five years.
Study Design:
- 445 patients enrolled
- 1:1 randomization versus Resolute Onyx DES
- Primary endpoint: TLF at 12 months (powered for non-inferiority)
- Secondary endpoints: Imaging by QCA/IVUS/OCT at 12 months
- Clinical follow-up to 5 years
Key Findings:
The clinical trial met its primary endpoint of Target Lesion Failure (TLF) non-inferiority at 12 months. The novel DynamX coronary bioadaptor achieved a very low 1.8% TLF rate compared to 2.8% for Resolute Onyx DES (p<0.001), as well as similar acute performance, acute lumen gain, and percent diameter stenosis at baseline. Two-year results show that the TLF curves of the BIOADAPTOR RCT diverge further after the 1-year follow-up in favor of DynamX, with significant difference in TLF and Target Vessel Failure (TVF) rates favoring the bioadaptor over DES.
And, for the first time seen in a coronary revascularization implant, the bioadaptor demonstrated normal pulsatility in the device treated segment as well as novel findings of plaque stabilization and regression, confirming restoration of vessel function at 12 months.
With pulsatility restored, more blood is able to flow with every heartbeat, bringing more oxygen deeper into the heart and helping alleviate symptoms associated with Coronary Artery Disease.
Primary endpoint: target lesion failure at 12 months vs. 2.8% for Resolute Onyx™ (p < 0.001)
Increase in blood flow with every heartbeat, as pulsatility returns
Plaque volume regression in lipid rich lesions vs. +10% for DES (p = 0.008)
Significant TLF reduction driven by lower CVD, TVMI, TLR
INFINITY-SWEDEHEART Clinical Trial
The INFINITY-SWEDEHEART study evaluates the safety and effectiveness of the DynamX coronary bioadaptor, the first coronary artery implant that adapts to vessel physiology, compared to Resolute Onyx drug-eluting stent in a large multi-center RCT in a broad patient population representative of every day clinical practice, including patients with Acute Coronary Syndrome (ACS).
It is the largest randomized trial of the DynamX coronary bioadaptor to date with 2,400 patients enrolled.
Study Design:
- Interventional Clinical Trial
- 2400 participants enrolled in Sweden
- Ages 18 – 85 years
- 1:1 randomization of eligible patients with DynamX : Resolute Onyx
- Intervention Model: Parallel Assignment
- Clinical follow up to 5 years
Primary Endpoint(s):
- Primary Device Oriented Clinical Endpoint is target lesion failure (TLF); a composite of cardiovascular death, target vessel myocardial infarction (TV-MI), or ischemia-driven target lesion revascularization (ID-TLR).
Powered Secondary Endpoints:
- Landmark analysis of TLF from 6 months to End of Follow-up
- Landmark analysis of TVF from 6 months to End of Follow-up
Key Findings:
INFINITY-SWEDEHEART is the largest and second RCT to confirm consistency of low and plateauing adverse event rates versus DES after the unlocking of the Bioadaptor at 6 months.
- DynamX bioadaptor met the primary endpoint
- Significant reduction and plateau in TLF (p=0.003) and TVF (p=0.008) from 6 to 12 months in prespecified landmark analyses, driven by reduction in all components, compared to DES
Primary Non-Inferiority Endpoint Met
DynamX
(N=1,189)
p-value <0.001
Difference: -0.41%
[-1.94%, 1.11%]
Resolute Onyx
(N=1,192)
Significant Reduction and Plateau in TLF Events After 6 Months
Favorable Reduction in CVD, TV-MI, ID-TLR After 6 Months
Furthermore, favorable outcomes for the bioadaptor in the high-risk subgroup of acute coronary syndrome, and consistent benefit in prespecified subgroups were observed.
TLF Landmarked at 6 Months in ACS
TLF Landmarked at 6 Months in LAD lesions
TLF Landmarked at 6 Months in Small Vessels (< 2.75 mm)
These results confirm the novel impact of the Bioadaptor in CAD treatment through its unique mechanism of action of restoring the hemodynamic modulation of a diseased artery.
DynamX Mechanistic Clinical Study
DynamX coronary bioadaptor demonstrated very good 36-month clinical outcomes, highlighted by the absence of target-vessel myocardial infarction and definite or probable device thrombosis, and only one target lesion revascularization up to 36 months.
Study Design:
- Multi-center, single-arm, mechanistic clinical study
- 50 patients at 7 international sites
- Treatment of single, de novo lesions
- Followed out to 30 days, 9 and 12 months, 2 and 3 years
Key Findings:
- Positive vascular remodeling demonstrated by a vessel area increase of 3% and a device area increase of 5%
- Cyclic pulsatility demonstrated by OCT with a difference in cross-sectional lumen area of 11% between systole and diastole
- 4 TLF out to 36 months: 3 deaths – investigational sites reported all as unrelated to device or procedure; only 1 clinically driven TLR
- Zero incidence of definite or probable device thrombosis
DESyne BDS Plus RCT
The DESyne BDS Plus Randomized Clinical Trial (RCT) is a prospective, multicenter, single-blind study evaluating the safety, effectiveness, and performance of world’s first triple-drug site-specific antithrombotic therapy (TRx) compared to DESyne X2 Drug-Eluting Coronary Stent System in the treatment of de novo native coronary artery lesions. The trial includes 202 patients across 14 sites in Europe, New Zealand and Brazil. An imaging subset of 58 patients had angiographic and optical coherence tomography (OCT) assessment completed in the first six months. Data collection will continue through three years.
Study Design:
- Prospective, multicenter, randomized
- 202 patients
- 60 patient imaging subset
- 14 sites
- Primary endpoint of TLF at 3 days or through hospital discharge
- Secondary endpoint of LLL at 6 months
Key Findings:
12-month results from DESyne BDS Plus RCT demonstrate the safety and effectiveness of site specific anti-thrombotic drug therapy
- No CVD, TV-MI, or stent thrombosis (definite/probable)
- Significantly lower TLF rate (2.1% vs. 9.3%, p=0.030) and TVF rate (2.1% vs. 10.3%, p=0.017) than the contemporary DES group in complex patients
- Drug pharmacokinetics results showed systemic subtherapeutic levels of the two anticoagulants through 7 days while maintaining therapeutic effect at the site of implant through 6 months
PINNACLE I Study for Intravascular Hertz Contact Lithotripsy
The PINNACLE I clinical study assesses the safety and performance of the LithiX Coronary Hertz Contact Intravascular Lithotripsy catheter to treat moderately to severely calcified coronary artery lesions by calcium fragmentation utilizing Hertz contact stress LithiX HC-IVL catheter.
Study Design:
- Multi-center, prospective, non-randomized, single arm
- Up to 60 patients
Imaging subgroup, n=32
- Primary Safety: MACE within 30 days (cardiac death, MI and TVR)
- Primary Efficacy: Clinical success defined as residual stenosis <50% after stenting with no evidence of in-hospital MACE
- Follow-up 30 days, 6 months
Key Findings:
PINNACLE I outcomes demonstrated the effectiveness and safety of LithiX™ Hertz Contact IVL for calcium fragmentation in a broad complex range of calcium morphologies
98.3% met primary safety and effectiveness endpoint (clinical success) and 100% main branch, in-lesion, post-stent angiographic success
- Low target lesion failure rate (1.7%) and no stent thrombosis (definite/probable) through 6-month clinical follow-up
- Acute gain of 1.60mm ± 0.48 final post-stent
- % diameter stenosis of 12.5% ± 4.5 final post-stent
- Procedure time of 59.5 minutes (40.5, 76.0)*
OCT analysis demonstrated effectiveness of the novel mechanism of action (N = 32; L = 32#)
Calcium fracture and fragmentation was identified in >90% of the calcified lesions with multiple fractures in 75% lesions
- Calcium fracture depth of 0.81mm ± 0.33 and fracture width 0.66mm ± 0.29
- Deep calcium fractures in eccentric and concentric calcified lesions post HC-IVL, confirming the mechanism of action of HC-IVL.
- Maximum continuous calcium arc of 263.4⁰ ± 77.1
- 31% presence of calcified nodules
- Optimal stent expansion of 100.9 ± 24.3%, 103.4 ± 25.6% and 96.7 ± 25.5% at minimum lumen area (MLA), maximum calcium site (MCS), and minimum stent area (MSA), respectively†
Optimal stent expansion (>90% average) achieved at MLA, MCS, MSA in the eccentric and concentric calcified lesions including those with calcium nodules
OCT Imaging Confirmed High Fracture Depth and Width Even in Complex Calcified Lesions
Calcium score 4
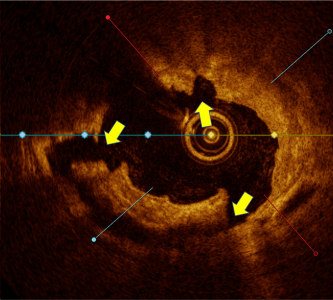
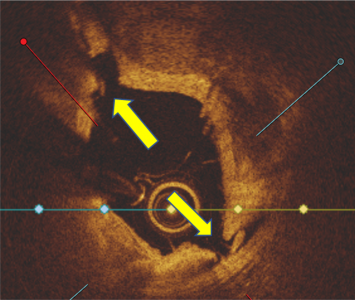
Calcium score 2, Nodule Present
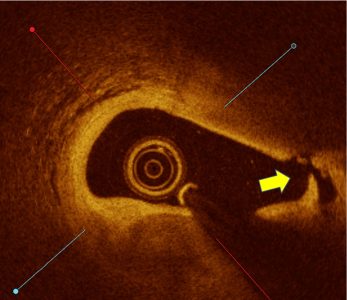
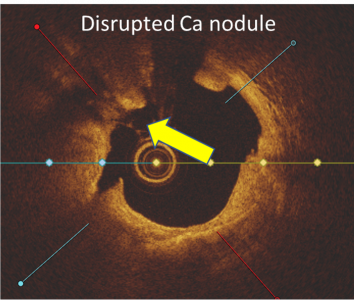
LithiX™ HC-IVL offers a safe, effective approach for calcium fragmentation to optimize stent implantation, without a need for external energy source and a simplified IVL workflow and learning curve
Note: *, Median (interquartile range). #, Subject has two target lesions with OCT analysis on one target lesion only. †, L=29 at MLA and L=30 at MCS and MSA.
PMN 1710 Rev E
