BREAKING
THROUGH
the Standard
BREAKING
THROUGH
the Standard
Hear from leaders in interventional cardiology as they discuss our unique technologies and clinical evidence. Or take a deep dive into how a technology works.
Interview with Dr. Shigeru Saito: BIOADAPTOR RCT results comparing novel DynamX Bioadaptor to Resolute Onyx DES. Dr. Saito explains the long-term risks associated with drug-eluting stents and the limitations of DES design. He shares key findings of the trial and how the unique design of the bioadaptor contributes to the significantly superior outcomes.
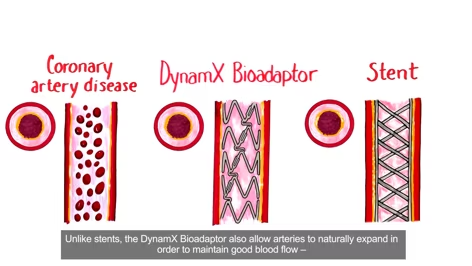
What is Coronary Artery Disease and Why Treating it with a Bioadaptor Matters? Learn how people are affected by coronary artery disease and its treatments. Understand disease treatment with stents and how they restore blood flow but restrict natural vessel motion and function, leading to major health issues. The DynamX coronary bioadaptor is a significant innovation that not only restores blood flow but also releases the vessel and allows it to pulsate more freely, increasing vital blood flow and returning it to a more natural state.
Interview with Dr. Stefan Verheye: BIOADAPTOR RCT results comparing novel DynamX Bioadaptor to Resolute Onyx DES. Dr. Verheye explains how the bioadaptor allows for restoration of vessel motion and function unlike the comparative DES. Pulsatility is restored resulting in significantly increased blood flow and lumen volume increased confirming adaptive remodeling when the vessel is uncaged. Findings also suggest potential benefits in plaque stabilization and regression.
PMN 1711 Rev A